How do stem cell feeding schedules affect quality?
Stem cell scientists are very familiar with the rigorous workload that is associated with culturing induced pluripotent stem cells (iPSCs). Feeding and maintenance schedules were designed at a time when figuring out how to grow stem cells was more important than understanding how to optimize research practices. Now that more time has been spent on developing more efficient ways of culturing, the landscape of feeding schedules is beginning to change. More media companies are developing weekend-free feeding options, but users are still trying to understand how changing their culture practices will affect the outcomes of their cultures.
How feeding schedules were developed
Over time, there has been a push to develop routine and reproducible feeding methods for stem cell research that include defined conditions. James Thomson and John Gearhart were among the first to isolate human embryonic stem cells and grow them in the lab. Their methods were feeder-dependent and required many reagents for proper cellular adhesion and proliferation. Since then, more defined culture media has been developed, allowing for feeder-free culture to be possible. However, this method of culture requires daily media changes to ensure the nutrients now provided by the media are replenished properly.
Many companies have developed their own variations of iPSC media. Some look to market towards more defined conditions while others look to market towards less of a feeding requirement. In recent years, more and more companies are looking to develop the perfect “weekend-free” medium. Scientists are enthusiastic about the potential to have their weekends back, and companies are eager for the labor savings that arise as a result.
However, current weekend-free solutions still have their limitations. They can require the use of a “double feed” the day before you’re looking to skip, meaning there is no savings in material costs. Some media variations contain mutant forms of protein that have detrimental effects on the cell quality, requiring scientists to spend additional time removing unwanted differentiation from cultures. These reasons discourage scientists from straying from their ingrained, daily practices. The question remains: is it possible to eliminate daily feeding in iPSC culture?
The reason daily feeding is required
Daily feeding has been an accepted method because it has proven to be the most successful to date. However, many people are unaware of why daily feeding is necessary. In order to create an effective non-daily feed culture method, it is important to understand how each media component impacts cells and why each needs frequent replenishment.
Many nutrients found in iPSC medium are growth factors that have a short half-life, often less than 5 hours. Because of this rapid degradation rate, the growth factors are contained in media at high concentrations (ex. 100 ng/mL), but their levels reach 0 ng/mL within a 24 hour period. Daily media feeds are required to replenish growth factors after they have degraded in culture.
One such growth factor that maintains the pluripotent nature of iPSCs is fibroblast growth factor 2 (FGF2). As FGF2 decays in culture media, iPSCs are encouraged to begin differentiating unwantedly. Cells undergo a cycle of differentiation as the FGF2 levels are added and then deteriorate with each feed. The daily fluctuations in FGF2 growth factor signals over time create a stressful environment for cell growth. Although daily feeding practices have worked best so far, these practices still do not create optimal conditions for cell growth. Nutrients are degrading at a rate not even daily replenishments can compete with.
Less frequent feeding creates better environments for cell growth
If culture conditions are adjusted to prevent the nutrients from degrading, cell quality can be improved with less frequent feeding. StemCultures has developed controlled-release growth factor technology to sustain growth factor levels over time. This technology encapsulates native protein into polymer carriers that slowly release the protein at constant levels over time. Figure 1 below shows typical growth factor levels when feeding cells daily compared to feeding twice per week with StemCultures’ DISC Devices.
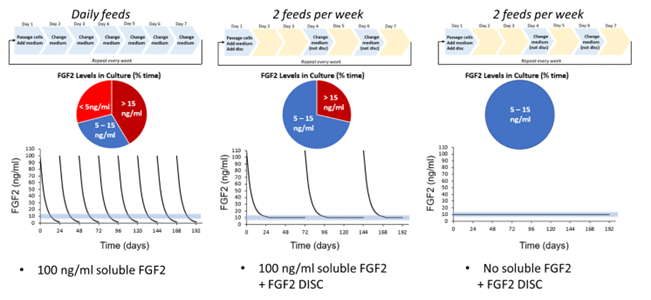
Figure 1: Graphical representation of growth factor levels when feeding daily compared to twice per week. With an FGF2 DISC, growth factor levels are sustained at the ideal level over several days.
In all cases using this technology, feeding schedules are reduced and results show increased proliferation, decreased unwanted spontaneous differentiation, and improve downstream differentiation efficiencies. Data supporting these claims can be seen in Figures 2-3 below. Increasing the time between medium changes allows natural metabolic biproducts to stay in the medium longer as well, more closely mimicking the natural mammalian environment. However, natural cell death also stays in the longer which can be alarming to some scientists. Our research has not shown any negative effects of increased debris on the quality of the cellular output.
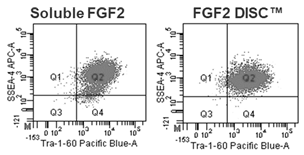
Figure 2: hPSCs grown with FGF2 DISCs show increased pluripotency markers SSEA4 and Tra-1-60 compared to daily media feeds of soluble FGF2 by flow cytometry.
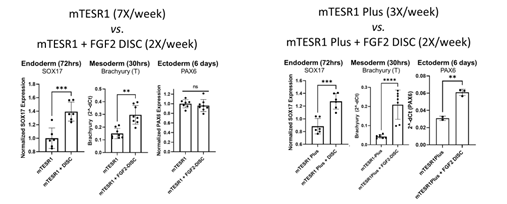
Figure 3: In these experiments, 3 hPSC lines were grown using 6 different methods: 3 different commercially available PSC media with or without an FGF2 DISC. Cultures with FGF2 DISCs had a reduced feeding schedule. After 3-4 passages, hPSCs were plated for endoderm, mesoderm, or ectoderm-directed differentiations. Cells were analyzed using qPCR for lineage-specific markers SOX17 (endoderm), Brachyury (mesoderm) and PAX6 (ectoderm).
Although it can be a large adjustment for scientists to change their feeding schedules, understanding how feeding schedules impact cell quality helps create the best cellular outcomes with as few feeds as possible. Controlled-release growth factor technology overcomes fluctuations in growth factor levels, allowing for the best environment for cell growth. This technology solves the need for daily feeding schedules. Scientists can make changes to their feeding schedules with improved cell quality outputs.