Properly maintained systems require direction and organization to operate effectively. Often, this is accomplished through accurate communication, with an initial instruction to start a specific process and a different instruction to end the process. This applies generally to all types of systems, but arguably the most complex example of this exists within biological systems. All our metabolic, growth, proliferation, and death pathways are triggered to start and stop based on instructional signals from the extracellular environment. Most often, these signals come in the form of growth factors, proteins or hormones designed to regulate cellular processes.
Typically, naturally grown mammalian cells have a readily available nutrient source from the body that supplies growth factors at the concentration required to maintain correct cellular signaling. Depending on the environment, this could be signaling for cell survival, growth, proliferation, or in some cases, cell death. Most often, an influx of a growth factor triggers survival and growth, and the removal of a growth factor triggers cell death signaling. When culturing in a laboratory setting, scientists are in control of the cell’s nutrient source. If the scientist misfeeds their cells, it could trigger undesired signaling pathways to begin, whether that be for proliferation or for death.
Stem Cell Growth Factors
To reduce the risk of misfeeding cultures, scientists have adapted regulated feeding schedules. Depending on the cell type in culture, this could require the scientist to add media into cell culture once per day, every day which can be a very tedious process. Daily media addition has been recognized as a necessity because growth factors degrade rapidly when added into culture media. This means growth factors are added into cultures at very high concentrations at the start of one day and degrade to negligible levels by the time scientists feed again the next day, creating large fluctuations in growth factor levels. A schematic of this process can be seen in Figure 1 below. This method makes it very difficult for scientists to know exactly what concentration of growth factors are present in their cultures at a given time, which in turn makes controlling cellular signaling nearly impossible. When cells are first fed, they have high levels of the growth factor which encourages growth and proliferation, but within a 24-hour period, the growth factor is no longer present and cell death pathways are triggered. This process repeats daily, putting the cells under a large amount of stress.
Figure 1: Schematic of fluctuating growth factor levels with traditional feeding schedules.
FGF2 and iPSCs
One growth factor commonly used to promote the proliferation of induced pluripotent stem cells (iPSCs) is fibroblast growth factor 2 (FGF2). FGF2 regulates the Mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway. This pathway is critical for cell signaling cascades, including those that regulate cell proliferation, differentiation, and cellular stress. In the case of iPSC culture, the addition of FGF2 triggers these pathways to start and its absence triggers these pathways to stop. Maintenance of these pathways at the most efficient level promotes proliferation and reduces unwanted spontaneous differentiation to keep stem cells from beginning to transition into other cell types.
Soluble FGF2 has a half-life of 4-5 hours, meaning it degrades rapidly after its initial injection into a cell culture well. As mentioned previously, this requires a daily feeding schedule and creates fluctuations in the amount of FGF2 that is available to the cells. The iPSCs then get mixed signals, don’t differentiate, differentiate, don’t differentiate, etc. over the course of a few days. This stressful signaling results in negative outcomes for scientist’s experiments. Not only does it take time to feed cells daily, but it also takes time for scientists to remedy any imperfections (excessive differentiation and cell death) that occur as a result.
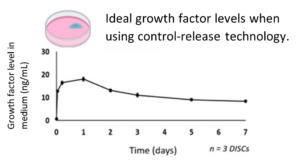
To replicate the natural mammalian environment most closely, it is important to find a way to provide consistent growth factors to cells grown in culture. Stabilizing growth factor levels eliminates the fluctuations in levels that comes with traditional feeding methods, keeping cell signals at a level that promotes the most efficient growth. Figure 2 below shows the ideal concentration of FGF2 over several days. With this method, cells no longer cycle between stages of life and death. Cells no longer struggle to survive. This means scientists get better and more efficient outputs for their experiments.
Figure 2: Ideal growth factor levels held constant over several days.
Luckily, StemCultures, LLC has become proficient in manufacturing sustained release growth factor technology for cell culture. StemCultures encapsulates growth factors so they release slowly over several days to match the ideal levels shown above. The company’s newest product line, FGF2 DISCs, have been shown to benefit iPSCs greatly. Figure 3 below shows how iPSC cultures are improved with stable growth factor levels, mimicking the natural environment. The cells were fed on a decreased schedule, only 3 times per week, and still showed improved quality. Notice the amount of unwanted spontaneous differentiation is drastically decreased and the colonies are tightly packed when a DISC is added into the culture media. FGF2 DISCs provide the proper growth factor levels needed to maintain the most efficient proliferation signaling.
Figure 3: Comparison of iPSCs grown with and without a DISC. Note: the cultures with a DISC were fed on a reduced schedule and the cultures without a DISC were fed daily.
All in all, the importance of control-release technology cannot be expressed enough. Consistency is the key ingredient that cells need to survive effectively. More effective cell growth results in more effective experimentation and more efficient advancements in regenerative medicine.
—-
Note: Opinions and accounts expressed herein are those of the author(s) or interviewee(s) and may not reflect those of StemCultures, its officers, or directors.