
StemCultures Training Video: Sustained Release Technology
Alongside the recent launch of several new products, StemCultures launched a training video on sustained release technology. Focusing on both general cell culture techniques as

Alongside the recent launch of several new products, StemCultures launched a training video on sustained release technology. Focusing on both general cell culture techniques as
Dr. Mark Post, head of physiology at Maastricht University, will debut the first stem cell burger the world has ever seen this Monday. Why? Dr.
When we are thirsty, nothing is better to me than an ice-cold glass of H20. Some people reach for something sweet, some like the carbonation, but

This past week, there was another instance of stem cell scientists creating hope. A team of stem cell scientists in Yokohama, Japan has grown tiny
In response to requests from our colleagues, we have released a trial size (1 mL) of StemBeads FGF2. Many stem cell scientists who engage in

Next week is 11th annual International Society for Stem Cell Research (ISSCR) meeting. This meeting is the largest forum for stem cell and regenerative medicine professionals from

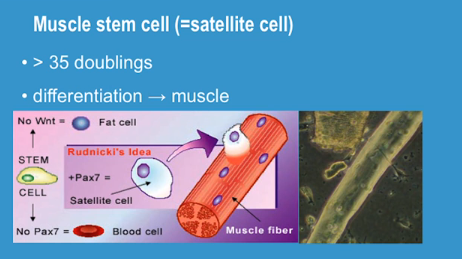
This is a post by Christine Miller, Research Assistant at Harvard University, Amy Wagers Lab: I think it is fair to say that most people

Very often I get that ever-popular question, “So what do you do?” Sometimes I wish I had an easy answer like car mechanic that explains

This is a post by StemCultures CEO Jeffrey Stern PhD, MD. When I think about the development of an organism, smooth edges, like the irresistible

Today, many can’t even remember how we lived before all of current technology, but we can maybe remember our eagerness to try them out when they were first introduced.